Energy Materials
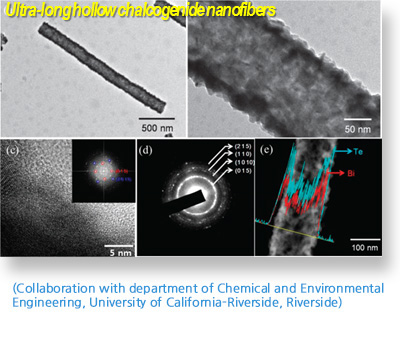
ThermoElectric Material
Thermoelectric materials are expected to provide a sustainable supply of energy. The dimensionless figure of merit, defined as ZT = (S2σ/k)T, plays a significant role in the conversion efficiency from thermal to electrical energy. Recently, thermoelectric materials\' research covers theoretical investigation, material design, and device assembling with a material form from bulk to low dimension structures. Most thermoelectric materials are characterized with various nanostructures from fine grains, dispersed particles, nano-inclusions to atomic defects, or segmented structures. We have our own approach to new thermoelectric device, which is to explore complex chalcogenide and intermetallic materials using newly developed solid state synthetic techniques such as electrochemical deposition method for these systems and using the applications such as thermoelectric materials and gas sensor.
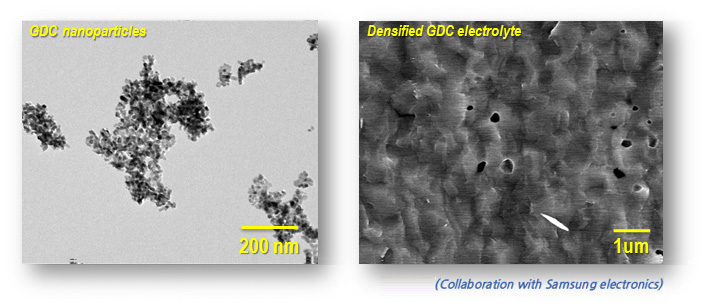
Solid Oxide Fuel Cell(SOFC)
Solid oxide fuel cells (SOFCs) have the promise to improve energy efficiency and to provide society with a clean energy producing technology. Solid oxide fuel cells are complex electrochemical devices that contain three basic components, a porous anode, an electrolyte membrane, and a porous cathode. For the electrolyte of SOFC, the required properties of electrolyte cover good ionic conductivity, no chemical interaction, good mechanical properties and thermal stability. We use the ceria-based materials GDC20 which has oxygen vacancy produced by 20 mol% Gadolinium, which has higher ionic conductivity and low activation energy than other solid electrolyte. For the good performance and reducing operation temperature, we fabricated the nanoparticles to get the high sintering density over 95% at low sintering temperature.
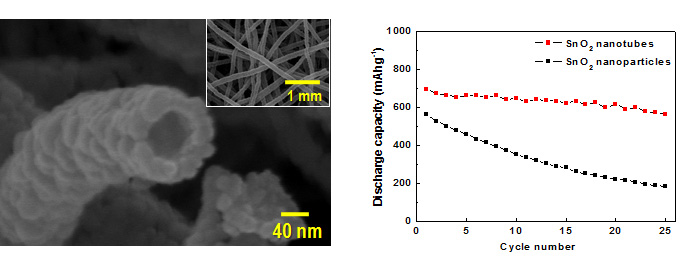
Lithium Ion Battery
Carbonous anode materials receive wide-range applications in commercialized LIBs, but the gravimetric and volumetric capacity of carbon materials is limited. The rapid development of electronic devices and EVs demands a much higher energy density as well as a higher power density and a smaller irreversible capacity for anodes. Tin oxide (SnO2) has high Li storage capacity by forming Li-containing alloys, but a large volume change is accompanied with the charging/discharging process, which results in a large inner stress and exfoliation of the electrode material and a rapid capacity fading upon prolonged cycles. Nanotubes can be largely alleviated due to their small grain size and large portion of surface atoms, and thus their cycling performance can be improved.
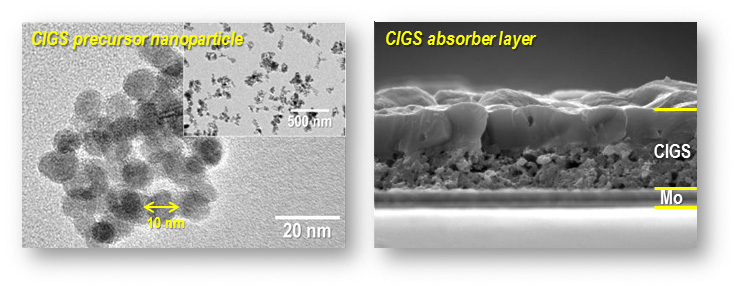
CIGS Solar Cell
The chalcopyrite semiconductor CuIn1-xGaxSe2 (CIGS) is an promising absorber material for thin film solar cells, because they have high conversion efficiency, direct band gap of 1.5 eV near a theoretical optimal value for high conversion efficiencies, high absorption coefficient(~105 cm-1) and high radiation stability. But they have been generally deposited by co-evaporation and sputtering methods that need a vacuum process with significant drawbacks such as expensive cost, loss of resource materials and restriction of large-scale production. To solve this problems, we synthesis the CIGS precursor nanoparticle by ultrasonic spray pyrolysis(USP) method. Synthesized CIGS precursor nanoparticle can be easily utilized as printing method that would be promising in terms of fast process, efficient use of the material and application of continuous roll-to-roll deposition.
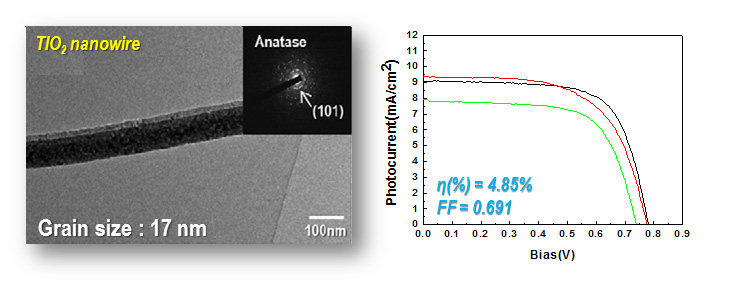
DSSC solar cell
Dye-sensitized solar cells (DSSCs) made from oriented, one-dimensional semiconductor nanostructures are receiving attention because direct connection of the point of photogeneration with the collection electrode using such structures may improve the cell performance. TiO2, generally known to one of the best semiconductor photocatalyst materials, can be electrospun and calcined in porous anatase nanowires, which expected to be applicable to high efficiency properties of DSSCs.
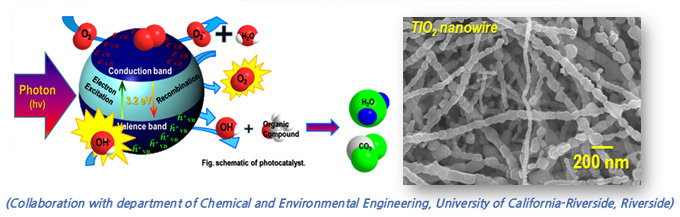
Photacatalyst
A promising approach for remediating air quality contaminants is to use photocatalyst that oxidize these compounds by accelerating photoreaction. It allows the oxidation of airborne VOCs and toxic organic matter into carbon dioxide and water at room temperature with UV or near-UV light source. It is required the high capability of catalytic decomposition in the reaction of the electron and hole by absorption of photonenergy. One-dimensional nanostructures with porous structure have sufficiently large surface area which is expected to increase reactant quantity onto the photocatalyst surface and the production of active oxygen species. In our lab, TiO2-based nanostructure fabricated by electrospinning process, which provides a simple method to produce mesoporous nanowire.