Abstract
Biological synthesis of gold nanostructures could potentially offer an environmentally friendly alternative to traditional chemical synthetic methods. During the last decades, various biomolecules, including amino acids, have been successfully used as reducing and capping agents to synthesize multi-shaped gold nanostructures. A grand challenge in this field is to increase our ability to control the size and shape of gold nanostructures formed precisely by systematic synthetic approaches based on the understanding of the mechanism for structural determination. In this study, using glycine as the model amino acid and chloroaurate (AuCl4-) ions as the precursor solution, we report the finding that the shape of the gold nanostructures synthesized showed a strong correlation with the speciation of gold complexes determined by the pH, precursor concentration and chloride concentration of the solvent system. The gold chloro-hydroxy speciation [AuClx(OH)4-x]- (with x = 0–4) influenced the shape of the gold nanostructures formed, with gold nanoplatelets, nanotriangles, nanokites and nanoribbons observed at x = 4, 3, 2 and 1, respectively, and spherical nanoparticles observed at x = 0. Glycine was found to play a role as a reducing agent, but no significant effect on the morphology was observed, indicating the dominance of gold chloro-hydroxy speciation in the structural formation. These results collectively provide synthetic considerations to systematically synthesize non-spherical to spherical biosynthesized gold nanostructures by controlling the speciation of [AuClx(OH)4-x]-.
Export citation and abstract BibTeX RIS
1. Introduction
Gold nanostructures present unique properties that enable wide applications in photonics, electronics, catalysis, bio/chemical sensing, drug delivery, and biological labeling [1–8]. Many of these applications require synthesis of monodisperse gold nanostructures with good control over sizes and shapes [9, 10]. Amongst many synthetic approaches, solution-based processes have attracted considerable attention because of its potential to offer significant cost-reduction per unit volume of material synthesized. A typical solution-based synthetic route involves the reduction of gold metal precursors in the presence of a reducing agent and stabilizer/capping agent [11–14]. The reducing agent (such as amines, borohydrides, etc) provides electrons to gold ions resulting in the nucleation of gold nanoclusters, which then grow either by intra-particle ripening or fast random attachment to the desired size. The stabilizer/capping agent acts as a size and shape regulator and stabilizes the gold nanostructures from aggregation or dissolution. Commonly used stabilizers/capping agents include organic surfactants such as hexadecyltrimethylammonium bromide and chloride (CTAB and CTAC), citrates, and polymer molecules such as poly (vinyl pyrrolidone) [15–18]. These have been successfully used to synthesize gold nanostructures in different shapes, including nanoparticles, nanowires, nanoribbons, and nanoplatelets, etc.
For the past decades, the growing interests in the use of gold nanostructures for biomedical applications have prompted extensive efforts to look for biocompatible reducing and capping agents [19]. A few examples of biocompatible reducing and capping agents include biomolecules such as proteins, peptides, amino acids, and deoxyribonucleic acid (DNA), etc. The gold nanostructures, when capped with biomolecules, have been found to be stable in biological matrices with little to noncellular toxicity [20]. For example, Khuller et al showed that gold nanoparticles capped by bovine serum albumin (BSA) are highly stable in high ionic strength media and causes no cytotoxicity, a stark difference to CTAB. Similarly, DNA has been used extensively as reducing and structure-designing agents for the synthesis of various gold nanostructures [21]. Amino acids, due to its structural simplicity and low cost, have emerged as an attractive biomolecule matrix for gold nanostructure synthesis. Sastry et al showed that amino acids could be used to synthesize anisotropic gold nanostructures [22]. Following this salient study, numerous articles have been published until recently on the synthesis of gold nanostructures using amino acids [23], including glycine, and their approach has been mainly focused on exploring new biomolecules to synthesize gold nanoparticles [24, 25]. Most of these works while elegantly discusses the synthesis of gold nanoparticles using amino acids, the influence of solvent parameters, such as pH, gold ion concentration and halide ion concentration on the shape of gold nanostructures formed is not well understood.
In this study, we focus on the complex behavior of the solvent system on amino acid-mediated synthesis of gold nanostructures. Particularly the role of pH, gold ion concentration, and chloride ion concentration in the glycine-mediated synthesis of gold nanostructures were investigated. Glycine was chosen because of its simple chemical structure amongst the amino acids. We systematically studied the effects of each chemical component concentration (glycine, proton, gold ion, and chloride ion) on the shape and kinetics of gold nanostructures formed. We demonstrate that it is the gold chloro-hydroxy speciation [AuClx(OH)4-x]- (with x = 0–4), which can be varied with gold ion concentration, pH, and the strength of chloride ions is predominantly responsible for the shape of gold nanostructures synthesized. For, x = 4, 3, 2, and 1, gold nanoplatelets, nanotriangles, nanokites, and nanoribbons were obtained, and spherical nanoparticles were obtained for x = 0. We further show that the value of x (and subsequently the nanostructure formed) can be tuned by changing the pH or by changing the chloride ion concentration for the same pH. Interestingly, glycine was found to play a role as a reducing agent, but no significant effect on the morphology was observed.
2. Materials and methods
2.1. Materials
Hydrochloroauric acid (HAuCl4) was purchased from Sigma Aldrich. Glycine, sodium chloride (NaCl), sodium hydroxide (NaOH), and hydrochloric acid (HCl) were purchased from Fisher Scientific. Nanopure water was prepared by using a Milli-Q system and was sterilized before use.
2.2. Synthesis of gold nanostructures
The experimental procedures were employed from our previous studies using peptide for the synthesis of gold nanostructures [26]. The reaction volume of the solution was kept at 1 ml, where the final concentration of HAuCl4 and glycine were varied between 0.5 and 0.18 mM. Prior to adding glycine, the pH of the HAuCl4 solutions was adjusted to pH 3.0 ± 0.1, 4.0, 5.0 ± 0.1, 6.5 ± 0.1 and 9.5 ± 0.1 by adding highly concentrated NaOH (5 M) and HCl (5 M). A high concentration of NaOH and HCl was used to minimize volume changes in the solutions. The mixed solutions were then placed in the dark at 37 °C for three days undisturbed. After the reaction, the solution was centrifuged at 12 000 rpm for 30 min to separate the synthesized gold nanostructures from the reaction solution. The supernatant was used for analyzing the amount of synthesized gold nanostructures. The gold nanostructures collected from the bottom of the centrifuge tube were structurally characterized.
2.3. Spectrophotometric analysis
The supernatant was diluted in 10% of HCl, and then the concentration of the gold ions was analyzed using atomic absorption spectrophotometer (AAnalyst800, Perkin Elmer, Inc.) at flame mode. The measured concentration of the remaining gold ions was subtracted from the initial gold concentration to calculate the amount of synthesized gold.
The optical properties of the gold nanostructures dispersed in nanopure water were measured with a Ultraviolet–visible–near infrared (UV–Vis–NIR) spectrophotometer (DU800, Beckman Coulter, Inc.) in the range of wavelengths from 400 to 1100 nm.
2.4. Structural characterization
Structural characteristics of the gold nanostructures were studied by using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The gold nanostructures were prepared on a silicon wafer by leaving a drop of a gold nanostructure suspension and letting it dry in ambient conditions. The structures were observed with SEM (XL30-FEG, Royal Philips Electronics, Inc.) at 15 kV of accelerating voltage.
For TEM imaging, the gold nanostructures dispersed in nanopure water were deposited onto carbon-coated Cu support grids and dried in ambient conditions. The structures were observed with TEM (JEM-2100F, JEOL, Ltd.) at an accelerating voltage of 200 kV. Selected area electron diffraction (SAED) was utilized to study the crystallographic orientation of the gold nanostructures.
3. Results and discussion
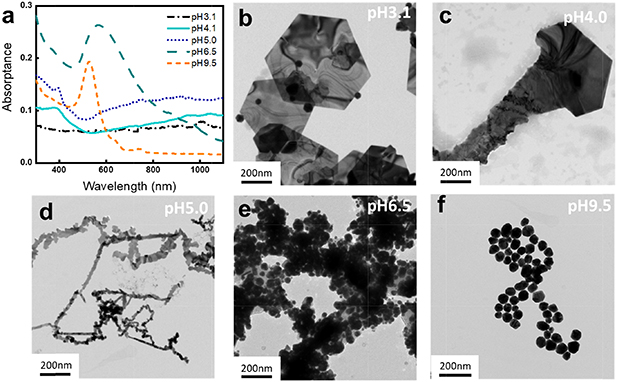
Aqueous HAuCl4 at 0.5 mM is pale yellow at pH 3, and as the pH increases, the solution color gradually fades and becomes transparent above pH 7. HAuCl4 mixed with 0.18 mM glycine and left in the dark at 37 °C for three days distinctively changes its color to transparent with shiny gold products at pH 3.1 and 4.0, dark blue, purple and pink at pH 5.0, 6.5, 9.5, respectively, indicating the formation of gold nanostructures in different shapes and sizes. (Figure SI1 available online at https://stacks.iop.org/NANO/31/455601/mmedia) Similar color transitions of the HAuCl4 solution after gold nanostructures formation were observed in previous studies [26, 27]. UV–Vis–NIR spectra of the gold nanostructures synthesized at different pH values (pH 3.1 to pH 9.5) are shown in figure 1(a). A single pronounced surface plasmon resonance (SPR) band at 527 nm, indicative of uniform spherical gold nanoparticles observed at pH 9.5. A broadening of SPR peak is observed for pH 4.1, which is an indication of larger size spherical gold nanoparticles (individual and/or aggregated) [28]. At lower pH values (pH 5.0, 4.0, and 3.1), however, no clear SPR peaks were observed in the measured wavelength range (300 ∼ 1100 nm). This could be either due to the formation of gold nanoparticles less than 2 nm or formation of microscopic two-dimensional gold nanostructures (such as platelets, ribbons, wires) with dipolar plasmon resonances red shifted to lower energies (>1200 nm) [27, 29]. TEM was used to further shed light on the gold nanostructures formed (figures 1(b)–(f)). Representative TEM images in figures 1(b)–(f) show the presence of a large number of hexagonal nanoplatelets at pH 3.1, nanokites, partial hexagonal geometry with serrated ribbon structures at pH 4.0, and serrated nanoribbons at pH 5.0. All structures observed at pH 3.1, 4.0, and 5.0 showed single-crystalline gold growing along (111). (Figure SI2) Spherical nanoparticle structure was confirmed for the nanostructures synthesized at pH 6.5 and 9.5: the cluster of broad nanoparticle size distribution attributed to the appearance of broader SPR band in the UV–Vis–NIR spectrum, for the products synthesized at pH 6.5 compared to the narrow band for the evenly sized particles synthesized at pH 9.5.
Figure 1. The UV–Vis spectra (a) and TEM images of gold nanostructures synthesized in 0.5 mM HAuCl4 and 0.18 mM glycine at pH 3.1 (b), 4.0 (c), 5.0 (d), 6.5 (e) and 9.5 (f) for 3 d at 37 °C.
Download figure:
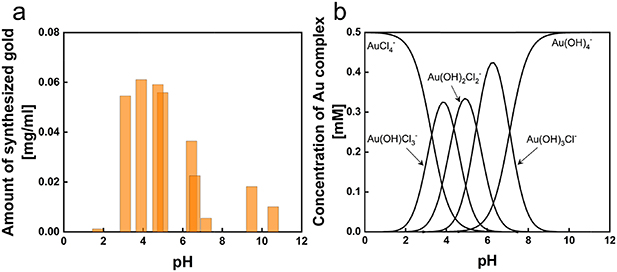
Standard image High-resolution imageThe amount of synthesized gold nanostructures (figure 2(a)) was measured by analyzing the remaining gold ions concentration in the supernatant that was separated from the synthesized gold by centrifugation. The largest amount of gold was synthesized at pH 3 ∼ 5, where relatively larger structures, such as nanoplatelet, nanokite, and nanoribbons, were synthesized, whereas the amount of synthesized gold was significantly low at pH above 7, where only spherical nanoparticles were synthesized. A negligible amount of synthesized gold was observed at pH below 2. The shape and amount of the synthesized gold nanostructures, interestingly, follow the speciation of [AuClx(OH)4-x)]- complexes attributed to the changes of pH. [AuClx(OH)4-x)]- complexes dominantly exist as [AuCl4]- at low pH; with increasing pH, chloride ligands are successively replaced by hydroxide ligands until the dominant species is [Au(OH)4]- above pH 8 (equations (1)–(4).
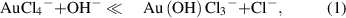
Figure 2. (a) The total amount of synthesized gold as a function of pH and (b) the concentration of [AuClx(OH)4-x)]- complex species in 0.5 mM HAuCl4 as a function of pH calculated with the equilibrium constants of AuClx(OH)4-x- species [25].
Download figure:
Standard image High-resolution image
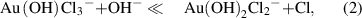
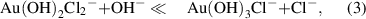
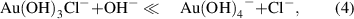
The concentration of AuClx(OH)4-x- as a function of pH in a 0.5 mM HAuCl4 solution (figure 2(b)) was calculated with the equilibrium constants reported by Nechayev et al [30]. At pH below 2, AuCl4- is the prevailing species; Au(OH)Cl3- is the most prevailing species at pH 4.0, Au(OH)2Cl2- at pH 5.0, Au(OH)3Cl- at pH 6.5 and the complexes become completely hydrolyzed to Au(OH)4- at pH above 10. The species of Au(OH)Cl3- and Au(OH)2Cl2- are correlated with the pH range in 3 ∼ 5 where the largest amount of gold synthesized, ∼0.06 mg ml−1, was observed with the formation of nanoplates, nanokites, and nanobelts. The species of Au(OH)3Cl- and Au(OH)4- correlate to pH 6.5 ∼ 9.5 that showed a low amount of gold synthesized below 0.02 mg ml−1 and associated with the formation of nanoparticles.
The speciation of [AuClx(OH)4-x]- complexes varies by the temperature, pH, the concentration of gold, and the concentration of Cl- [31–33]. Especially, excess Cl- in HAuCl4 solution stabilizes the [AuClx(OH)4-x]- species shifting the hydrolysis to occur at higher pH values. To further probe the correlation between the [AuClx(OH)4-x]- speciation and the shape of the synthesized nanostructures, the synthesis was replicated in the same condition with excess Cl-. The [AuClx(OH)4-x]- speciation with 0.5 M NaCl was calculated and shown in figure 3(a). The addition of 0.5 M NaCl shifts the speciation of [AuClx(OH)4-x]- complexes to higher pH values, approximately by 2.5, while remaining similar trends in the amount of each species. The overall amount of synthesized gold with 0.5 M NaCl (figure 3(b)) was significantly decreased but had similar trends compare to the results without additional NaCl showing the largest amount of synthesized gold in the pH range where Au(OH)Cl3- and Au(OH)2Cl2- exist predominantly. The structures of the synthesized nanostructures also follow the structure-speciation correlation previously observed without excess Cl- as shown in the SEM images (figure 3(d)): nanoplatelets were synthesized at pH 4.7 and 5.8, nanokites at pH 6.6 and 7.5, and nanoparticles beyond pH 10.1(figure 3(d)). These results corroborate the correlation between the [AuClx(OH)4-x]- complexes and the shape of nanostructures. The overall decrease of the amount of synthesized gold with excess Cl- is attributed to the state of glycine as a function of pH. Glycine exists as a zwitterion near its isoelectric point (pI 6.0) where the amine group in glycine is protonated (–NH3+), and the carboxyl group is deprotonated (–COO-) thus the net charge becomes zero (figure 3(c)). At pH below the pI of glycine, the net charge of glycine is positive due to the protonated α-carboxyl group (–COOH) and at pH above the pI of glycine, the net charge of glycine is negative due to the deprotonated amine group (–NH2). Without excess Cl-, the most reactive species, Au(OH)Cl3- and Au(OH)2Cl2-, exist mostly below pH 6 where positively charged glycine is electrostatically attracted to [AuClx(OH)4-x]- complexes, however, with excess Cl-, Au(OH)Cl3- and Au(OH)2Cl2- most exist in pH above 6 where glycine is negatively charged repulsing the [AuClx(OH)4-x]- complexes [34, 35]. The amount of synthesized gold is negligible in the pH range above 10 and below 2. In the pH range above 10, the amine-group of glycine is completely deprotonated (pH > 10) inducing electrostatic repulsion between glycine and [AuClx(OH)4-x]- complexes.
Figure 3. (a) The calculated distribution of [AuClx(OH)4-x]- complexes in 0.5 mM HAuCl4 shifts to higher pH ranges with (solid line) and without (dash line) addition of 0.5 M NaCl. (b) The amount of synthesized gold in 0.5 mM HAuCl4 and 0.18 mM glycine with (blue) and without (orange) addition of 0.5 M NaCl (c) The titration curve of glycine showing the equivalent of hydrogen protonated and deprotonated from glycine as a function of pH. (d) The SEM images of the synthesized nanostructures in the solution with an additional 0.5 M NaCl. Inset scale bar: 100 nm.
Download figure:
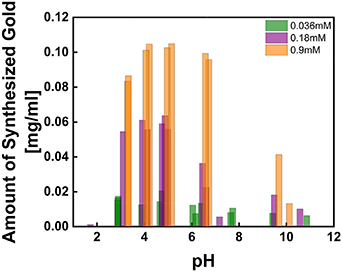
Standard image High-resolution imageThe effect of the glycine concentration was studied by varying the concentration from 0.036 to 0.18, and 0.9 mM in 0.5 mM HAuCl4. The solution was incubated in the same condition, 72 h of incubation at 37 °C. With 0.036 mM glycine, the yield of the amount of synthesized gold in the initial amount of gold is in the range of 12.5% ∼ 20.8% from pH 3 to pH 5, and 6.4% ∼ 13.6% above pH 7 (figure 4). It is to be noted that what we report here is the absolute yield—total gold nanostructures formed for given gold salt concentration—and not qualitative yield as reported for most amino acid-mediated synthesis [36]. The amount of glycine relates to the reduction rate, but no significant contributions in controlling the morphologies were observed.
Figure 4. The amount of synthesized gold in 0.5 mM HAuCl4 with 0.036, 0.18, and 0.9 mM glycine shows an increased reduction rate with respect to the increased amount of glycine.
Download figure:
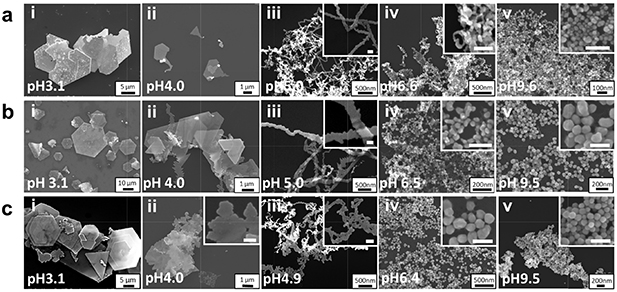
Standard image High-resolution imageSEM images in figure 5 show the structural progression with respect to the change of glycine concentration. In all glycine concentrations, hexagonal platelet structures were formed at pH 3; however, the platelet structure was incompletely grown at 0.036 mM, whereas the platelets were in fully grown structures at 0.18 mM and overly grown into multi-layered spiral structures at 0.9 mM. The concentration of glycine had more influence on the growth of the nanostructures rather than on the nucleation process, indicating that the nucleation process is independent of the amount of glycine. At pH 4, nanokite structures with smaller hexagonal or triangular platelets and tails were formed at 0.036 mM of glycine compare to the nanokites formed at 0.18 mM. Nanoplatelet structures overly grown on the sides of the kite structures in random fashion were observed at 0.9 mM of glycine. At pH 5, narrow serrate nanoribbons were synthesized at 0.036 and similar but wider structures at 0.18 mM of glycine. With 0.9 mM glycine at pH 5, the nanoribbons were randomly grown without the serrated edges. Nanostructures resembling tadpoles were synthesized at pH 6.5 in 0.036 mM of glycine, nanoparticles with uneven morphology in 0.18 mM glycine, and relatively evenly size-distributed nanoparticles in 0.9 mM. Nanoparticles were synthesized at pH 9.5 regardless of the concentration of glycine. However, the uniformity of the nanoparticles was improved with higher glycine concentration.
Figure 5. The SEM images of gold nanostructures synthesized in 0.5 mM HAuCl4 with 0.036 mM (a), 0.18 mM (b), and 0.9 mM (c) of glycine. The structures in the pH ranges of 3 ∼ 7 showed an overgrowth of structures as the concentration of glycine increased. Inset scale bar: 100 nm.
Download figure:
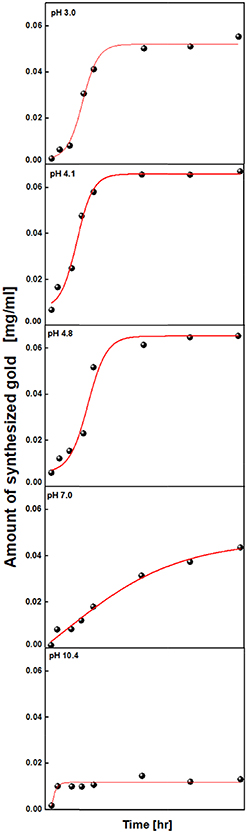
Standard image High-resolution imageThe dependence of reaction time on the shape and amount of gold nanomaterials was studied. The time courses of the amount of synthesized gold (figure 6) showed a similar characteristic that has a dramatic increase in the amount of synthesized gold in the middle of the reaction, which suggests the involvement of autocatalysis [37, 38]. After 24 h, the reaction almost reached to plateau state indicating the termination of the reaction except for pH 7.0, which gradually increased until 72 h of the reaction and pH 10.4, which already reached a plateau state in the first 6 h. The time courses of the amount of synthesized gold with different glycine concentration at a fixed pH 3.1 were also studied.
Figure 6. Time-evolved studies of the glycine-mediated synthesis of gold nanostructures. The Boltzmann sigmoid fit of the amount of synthesized gold as a function of time at pH 3.0, 4.1 and 4.8 shows the nucleation regime in the first 30 h followed by a linear growth regime at pH 3.0, 4.1 and 4.8.
Download figure:
Standard image High-resolution imageFigure 6 shows the evolution of gold nanostructures synthesized as a function of time. The sigmoidal curves at pH 3.0, 4.1, and 4.8 show the process of nucleation in the first 30 h and growth in the linear region by random attachment and intraparticle ripening, indicating the growth of particles into larger structures. A long nucleation regime was observed for pH 7, with the amount of synthesized gold gradually increasing for 90 h. At pH 10.4, a rapid nucleation process was observed in the first few hours. However, most of the gold precursors remained unreacted, indicating the termination of the reaction. Particle and irregular wire structures were dominantly grown until 3 h of incubation, wide ribbon, and aggregated wire structures during 3 ∼ 6 h and nanoribbon structures with a small platelet during 9 h of incubation. We believe that such structural variation only at pH 4.1 may be due to the existence of the equimolar ratio of the two complex species (Au(OH)2Cl2- and Au(OH)Cl3-). Any slight change of the pH during the incubation ends up with the variation of the molar ratio, and therefore, the structural variation occurs.
4. Conclusions
The structural determination of gold nanostructures strongly depends on the speciation of [AuClx(OH)4-x]- complexes resulted from the changes in pH and the concentration of excess chloride. Among the gold complex species, AuCl(OH)3- and AuCl2(OH)2- were linked to the largest amount of synthesized gold regardless of the pH range. The amount of synthesized gold is convincingly related to the ionic interactions between the gold complex and glycine, resulting in a large amount of synthesized gold at pH ranges where glycine and gold complexes have electrostatic attractions. The results from the varying glycine concentration show that the nucleation process is independent of the concentration of glycine, and in relation to the transient kinetics, the particle growth follows LaMer mechanism[39] at pH 3.1, 4.0, and 5.0 that maximizes the particle sizes. The role of glycine was mainly a reducing agent providing electrons for the gold reduction, and nearly no roles as a capping agent, directing the growth of gold nanostructures, were observed. This study clearly shows that the [AuClx(OH)4-x]- speciation plays an important role in determining the structural formation of gold nanostructures and provides a set of synthetic considerations for controlling the shape of gold nanostructures. Further studies on the mechanism of shape formation by [AuClx(OH)4-x]- complexes associated with their binding affinity may contribute to simplifying synthetic approaches and can be expanded to investigate the shape evolution of other noble metal nanostructures.
Acknowledgments
This research was supported by the Future Materials Discovery Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (NRF-2016M3D1A1027836).
Author contributions:
Dr. Joun Lee conducted most of the experiments and drafted the manuscript. Drs. Syed Mubeen and Sanggon Kim assisted the data analysis and wrote the manuscript. Profs. Ashok Mulchandani, Wilfred Chen, and Nosang V. Myung came up with the concept and supervised these students. Prof. Yongho Choa conducted TEM analysis. Dr. Joun Lee was a joint Ph. D. student of Profs. Mulchandani, Chen, and Myung.